|
16.12.2021 18:55:00
|
Sky wins FDA clearance to market the geko™ device for venous insufficiency and ischemia - a therapy area sorely in need of innovation.
DARESBURY, England, Dec. 16, 2021 /PRNewswire/ -- Medical devices company, Sky Medical Technology Ltd, has today announced U.S. Food and Drug Administration (FDA) 510(k) clearance to market the geko™ device for increasing microcirculatory blood flow in lower limb soft tissue of patients with venous insufficiency and/or ischemia.
The geko™ device is the first electrical neuromuscular stimulator of its kind to be cleared for increasing microcirculatory blood flow and adds to Sky's prior FDA clearances for edema reduction and for stimulation of the calf muscles to prevent venous thrombosis (blood clots) in both surgical and non-surgical patients.
Venous insufficiency and ischemia relate to reduced blood flow in the veins and arteries. This can lead to lower extremity edema, skin changes, and discomfort. Venous insufficiency can progress to chronic venous insufficiency (CVI), a serious condition attributed to diminished quality of life and loss of work productivity. In most cases, the cause is incompetent valves. Each year approximately 150,000 new patients are diagnosed with chronic venous insufficiency, and nearly $500 million is used in the care of these patients. If venous insufficiency and ischemia are left untreated, progressive to CVI can lead to post-phlebitic syndrome and venous leg ulcers1.
Three recent published studies, two led by Das2,3, the other led by Bosanquet4 demonstrate geko™ device generated microcirculatory blood flow increase in the lower limbs of patients with venous insufficiency and ischemia. Both studies measured the increase using Laser Speckle Contrast Imaging, a non-invasive technique that measures blood flow.
CEO and Founder Bernard Ross said, "Achieving this latest 510(k) clearance is a significant milestone for Sky that will allow us to initiate a controlled market release of the geko™ device, to address venous insufficiency and ischemia in the first instance – a therapy area sorely in need of innovation. With this 510(k) and in partnership with leading US clinicians we can now press ahead to redefine the way vascular related conditions can be treated."
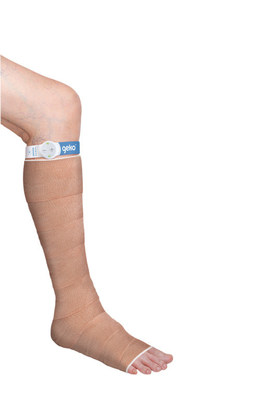
The geko™ device is a non-invasive, easy to use, wearable therapy device. The size of a wristwatch and worn at the knee, the disposable device gently stimulates the common peroneal nerve activating the calf and foot muscle pumps, resulting in increased blood flow in the deep veins of the calf5, at a rate equal to 60%6 of walking without a patient having to move. The device operates without external pressure to the leg and allows complete mobility.
The company is headquartered in the UK and backed by leading international investors in both healthcare and technology. The company is in the process of submitting further FDA 510(k) applications to expand its claims related to blood flow increase to address venous insufficiency.
References
1. Patel SK et al. Venous Insufficiency. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan. 2021 Aug 4.
2. Das et al. Neuromuscular stimulation of the common peroneal nerve increases arterial and venous velocity in patients with venous leg ulcers. Int Wound J. 2020;1–7. DOI: 10.1111/iwj.13510. September 2020.
3. Das et al. Microcirculatory changes in venous leg ulcers using intermittent electrostimulation of common peroneal nerve, Journal of Wound Care. Vol 30, No 2. February 2021
4. Bosanquet DC et al. Microcirculatory Flux and Pulsatility in Arterial Leg Ulcers is Increased by Intermittent Neuromuscular Electrostimulation of the Common Peroneal Nerve. Published by Elsevier Inc. Open access article under the CC BY-NC-ND license. Manuscript received: May 14, 2020; manuscript accepted: July 9, 2020; published online.
5. Nicolaides A, Griffin M. Measurement of blood flow in the deep veins of the lower limb using the geko™ neuromuscular electro-stimulation device. Journal of International Angiology August 2016-04.
6. Tucker A et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. The International journal of angiology: official publication of the International College of Angiology, Inc. 2010 Spring;19(1): e31-7.
About Sky Medical Technology Ltd and Firstkind Ltd
Sky Medical Technology, the parent of Firstkind Ltd, is a UK-based medical devices company. Through its innovative mechanism of non-invasive neuromuscular electrostimulation, Sky has developed a ground-breaking NMES technology platform, OnPulse™, embedded in its industry-leading product, the geko™ device. The company develops a range of products tailored to the needs of different medical application areas, selling both direct and through strategic partnerships or distributors in each major clinical area. Clinical areas of interest include DVT prevention, the treatment and reduction of edema and venous insufficiency. The goal in each pathway is to partner with healthcare professionals to improve clinical outcomes and patient care whilst saving health system resources.
Sue Davenport, +44 7771 667 170, sue.davenport@firstkindmedical.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/sky-wins-fda-clearance-to-market-the-geko-device-for-venous-insufficiency-and-ischemia--a-therapy-area-sorely-in-need-of-innovation-301446803.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/sky-wins-fda-clearance-to-market-the-geko-device-for-venous-insufficiency-and-ischemia--a-therapy-area-sorely-in-need-of-innovation-301446803.html
SOURCE Sky Medical Technology Ltd.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!