|
14.05.2018 22:02:00
|
Pfenex Announces Positive Top-Line PF708 Study Results in Osteoporosis Patients, On-Track for Submission of New Drug Application in Third Quarter 2018
SAN DIEGO, May 14, 2018 /PRNewswire/ -- Pfenex Inc. (NYSE American: PFNX), a clinical-stage development and licensing biotechnology company focused on leveraging its Pfēnex Expression Technology® to improve protein therapies for unmet patient needs, today announced top-line results from its PF708-301 study, which showed comparable overall profiles between PF708 and Forteo® after 24 weeks of daily injection in osteoporosis patients. Pfenex expects to submit an New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) in the third quarter of 2018.
"We are pleased with the results of the PF708-301 study, which show comparable overall profiles between PF708 and Forteo," said Eef Schimmelpennink, Chief Executive Officer of Pfenex. "We expect that these results from the PF708-301 study, along with the previously announced bioequivalence findings from the PF708-101 study in healthy subjects, will support the PF708 NDA submission. We are on-track for submission to the FDA in the third quarter of 2018, with a potential commercial launch in the United States as early as the third quarter of 2019, subject, of course, to FDA approval of the application."
PF708 is a teriparatide drug candidate that is being developed as a therapeutic equivalent to Forteo, which is approved and marketed by Eli Lilly for the treatment of osteoporosis patients at a high risk of fracture. PF708 is being developed pursuant to the 505(b)(2) regulatory pathway in the United States and references Forteo as the Reference Listed Drug.
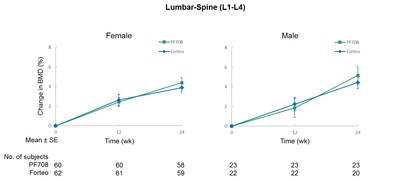
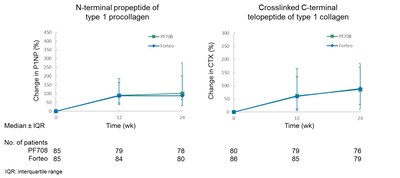
The PF708-301 study enrolled a total of 181 patients, with 90 patients receiving PF708 and 91 receiving Forteo. There were 82 patients who completed the study in the PF708 treatment group, compared with 81 patients in the Forteo treatment group. The primary study endpoint was anti-drug antibody (ADA) incidence after 24 weeks of drug treatment. The secondary study endpoints included mean percentage changes in lumbar-spine bone mineral density (BMD) and median percentage changes in bone turnover markers (BTM) after 24 weeks of drug treatment, as well as pharmacokinetic (PK) parameters for up to four hours after the first dose. Safety study endpoints were incidences of adverse events (AE) and serious adverse events (SAE).
There were two PF708-treated patients and two Forteo-treated patients that developed ADA during the study. These low rates of immunogenicity are consistent with historical Forteo results (~3%) in postmenopausal osteoporosis patients. At Week 24, there were two ADA-positive findings for PF708 compared with none for Forteo, but the difference was not statistically significant. The ADA findings in the two PF708 patients were low in titer and resolved during follow-up. One of the two patients had in vitro neutralizing activity transiently detected at Week 4. However, pharmacological activity, as assessed by increases in BMD and BTM, was observed during the study for this patient. There were no apparent safety issues or abnormal serum calcium levels related to ADA or neutralizing antibody findings. These findings are consistent with observations in follow-on biologics and biosimilars approved in the United States, with almost all of the products demonstrating an ADA treatment difference of less than 5% in comparative patient studies. The overall ADA results are shown in Table 1, and individual titer results for all ADA-positive patients are shown in Table 2 (below).
PF708 and Forteo demonstrated comparable effects on lumbar-spine BMD, P1NP (N-terminal propeptide of type 1 procollagen), which is a marker of bone formation and CTX (cross-linked C-terminal telopeptide of type 1 collagen), which is a marker of bone resorption. There were no statistically significant differences in any of these parameters between PF708 and Forteo. The lumbar-spine BMD results are shown in Figure 1 (below), and BTM results are shown in Figure 2 (below).
There were no significant imbalances in AE incidences or severity profiles between PF708 and Forteo. Treatment-emergent AE and SAE profiles are shown in Table 3 (below), and the severity of treatment-emergent AEs is shown in Table 4 (below).
"The PF708-301 study assessed PF708 and Forteo across multiple endpoints in both female and male osteoporosis patients and showed comparable overall profiles. We believe the PF708-301 and PF708-101 study results meet the requirements for demonstrating clinical safety, effectiveness and bioequivalence," said Hubert C. Chen, MD, Chief Medical and Scientific Officer of Pfenex. "We look forward to making our NDA submission to the FDA and engaging in discussion with the agency over the course of the review process."
Conference Call Information
The Pfenex management team will host a conference call and a live webcast today at 4:45 p.m. ET/1:45 p.m. PT to discuss the top-line results from the PF708-301 study. Please use the information below to access the call.
Monday, May 14, 2018 @ 4:45 p.m. Eastern Time/1:45 p.m. Pacific Time | |
US Toll Free: | 866-376-8058 |
International: | 412-542-4131 |
Webcast: | |
A replay of the call will be available through May 21, 2018: | |
US Toll Free: | 877-344-7529 |
International: | 412-317-0088 |
Replay Access Code: | 10120499 |
Webcast: | |
About Pfenex Inc.
Pfenex Inc. is a clinical-stage development and licensing biotechnology company focused on leveraging our Pfēnex Expression Technology® to improve protein therapies for unmet patient needs. Using the patented Pfēnex Expression Technology platform, the company has created an advanced pipeline of therapeutic equivalents, vaccines, biologics and biosimilars. The company's lead product candidates are PF708, a therapeutic equivalent candidate to Forteo® (teriparatide) for the treatment of osteoporosis, and our novel anthrax vaccine candidates, Px563L and RPA563, funded through an advanced development contract with the U.S. government. In addition, Pfenex is developing hematology/oncology products, including PF743, a recombinant crisantaspase, and PF745, a recombinant crisantaspase with half-life extension technology, in collaboration with Jazz Pharmaceuticals. Furthermore, the company's pipeline includes biosimilar candidates to Lucentis® and Neulasta®.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements because they contain words such as "may," "will," "should," "expects," "plans," "anticipates," "could," "intends," "target," "projects," "contemplates," "believes," "estimates," "predicts," "potential" or "continue" or the negative of these words or other similar terms or expressions that concern Pfenex's expectations, strategy, plans or intentions. Forward-looking statements in this press release include, but are not limited to, statements regarding the future potential of PF708, including the expectation that Pfenex will submit an NDA in the third quarter of 2018; that the PF708-301 study results will support the NDA submission; and the timing of a potential commercial launch. Actual results may differ materially from those indicated by these forward-looking statements as a result of the uncertainties inherent in the clinical drug development process, including, without limitation, challenges in successfully demonstrating the efficacy and safety of product candidates; the pre-clinical and clinical results for product candidates, which may not support further development of product candidates or may require additional clinical trials or modifications of ongoing clinical trials or regulatory pathways; challenges related to commencement, patient enrollment, completion, and analysis of clinical trials; Pfenex's ability to obtain additional funding to support its business activities and establish and maintain strategic business alliances and new business initiatives; Pfenex's dependence on third parties for development, manufacture, marketing, sales and distribution of products; unexpected expenditures; and difficulties in obtaining and maintaining intellectual property protection for product candidates. Information on these and additional risks, uncertainties, and other information affecting Pfenex's business and operating results is contained in Pfenex's Quarterly Report on Form 10-Q for the quarter ended March 31, 2018 and in Pfenex's subsequent reports filed with the Securities and Exchange Commission. The forward-looking statements in this press release are based on information available to Pfenex as of the date hereof, and Pfenex disclaims any obligation to update any forward-looking statements, except as required by law.
Pfenex investors and others should note that we announce material information to the public about the Company through a variety of means, including our website (http://www.pfenex.com/), our investor relations website (http://pfenex.investorroom.com/), press releases, SEC filings, public conference calls, corporate Twitter account (https://twitter.com/pfenex), Facebook page (https://www.facebook.com/Pfenex-Inc-105908276167776/timeline/), and LinkedIn page (https://www.linkedin.com/company/pfenex-inc) in order to achieve broad, non-exclusionary distribution of information to the public and to comply with our disclosure obligations under Regulation FD. We encourage our investors and others to monitor and review the information we make public in these locations as such information could be deemed to be material information. Please note that this list may be updated from time to time.
Table 1. Study PF708-301 Overall Anti-Drug Antibody Results
Time (wk) | PF708 | Forteo | P value | ||
0 | 0/90 | 0% | 0/91 | 0% | 1.00 |
1 | 1/87 | 1.2% | 0/90 | 0% | 0.49 |
4 | 1/86 | 1.2% | 0/89 | 0% | 0.49 |
12 | 2/82 | 2.4% | 2/86 | 2.3% | 1.00 |
24 | 2/81 | 2.5% | 0/81 | 0% | 0.50 |
24-week overall | 2/87 | 2.3% | 2/90 | 2.2% | 1.00 |
Table 2. Study PF708-301 Anti-Drug Antibody Titer Results for Individual Patients
Time (wk) | PF708 Patient 1 | PF708 Patient 2 | Forteo Patient 1 | Forteo Patient 2 |
0 | Neg | Neg | Neg | Neg |
1 | 1:1 | Neg | Neg | Neg |
4 | 1:1* | Neg | Neg | Neg |
12 | 1:1 | 1:1 | 1:8 | 1:2 |
24 | 1:1 | 1:1 | Neg | Neg |
Follow-up | Neg | Neg | N/A | N/A |
*In vitro neutralizing activity detected; pharmacological activity, as assessed by changes in BMD and BTM, was observed during the study for this patient. |
Antibody titer measures how much ADA is present in a positive sample. A value of 1:1 is the lowest possible finding, whereas a value of 1:8 represents an 8-fold increase. |
Neg: negative; N/A: not applicable |
Table 3. Study PF708-301 Treatment-Emergent Adverse Event Profiles
Number and Percent of Patients with: | PF708 | Forteo | ||
Any AE | 75 | 83.3% | 73 | 80.2% |
Any SAE | 6 | 6.7% | 8 | 8.8% |
Any treatment-related AE | 48 | 53.3% | 45 | 49.5% |
Any AE leading to early withdrawal | 3 | 3.3% | 5 | 5.5% |
Any AE leading to death | 0 | 0% | 0 | 0% |
AE: adverse event; SAE: serious adverse event |
Table 4. Study PF708-301 Severity of Treatment-Emergent Adverse Events
Grade | Grade | Grade | Grade | Total | |||
All | PF708 | 169 | 84 | 6 | 2 | 261 | |
Forteo | 203 | 61 | 9 | 3 | 276 | ||
Grade | Grade | Grade | Grade | Total | |||
Injection | PF708 | 36 | 1 | 0 | 0 | 37 | |
Forteo | 33 | 1 | 0 | 0 | 34 | ||
Severity of AEs was assessed according to the Common Terminology Criteria for Adverse Events, Version 4.03 |

![]() View original content with multimedia:http://www.prnewswire.com/news-releases/pfenex-announces-positive-top-line-pf708-study-results-in-osteoporosis-patients-on-track-for-submission-of-new-drug-application-in-third-quarter-2018-300647961.html
View original content with multimedia:http://www.prnewswire.com/news-releases/pfenex-announces-positive-top-line-pf708-study-results-in-osteoporosis-patients-on-track-for-submission-of-new-drug-application-in-third-quarter-2018-300647961.html
SOURCE Pfenex Inc.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu Pfenex Incmehr Nachrichten
| Keine Nachrichten verfügbar. |