|
02.12.2018 18:30:00
|
NewLink Genetics Presents Encouraging Updated Phase 1 Data with Indoximod Plus Chemotherapy in Frontline AML in an Oral Session at 2018 ASH Annual Meeting
NewLink Genetics Corporation (NASDAQ:NLNK) announced that updated Phase 1 data evaluating indoximod plus standard-of-care chemotherapy for the treatment of adult patients with newly diagnosed acute myeloid leukemia (AML) were presented today by Ashkan Emadi, MD, PhD, Professor of Medicine and Associate Director for Clinical Research, University of Maryland Greenebaum Comprehensive Cancer Center, in an oral session today at the 60th American Society of Hematology (ASH) Annual Meeting in San Diego, CA, from 9:30AM – 11:00AM PT, in Grand Hall B, Manchester Grand Hyatt.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20181202005047/en/
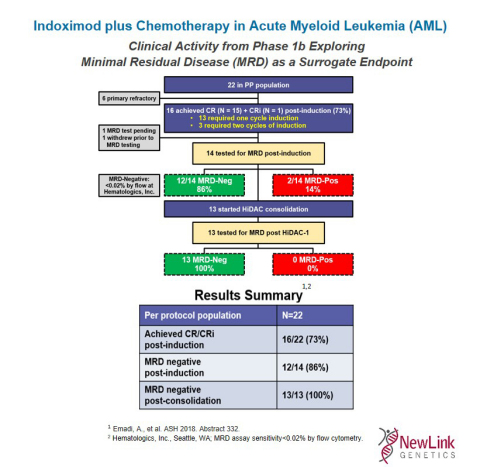
Indoximod plus Chemotherapy in Acute Myeloid Leukemia (AML) (Graphic: Business Wire)
This Phase 1 trial evaluated the initial safety and preliminary evidence of clinical activity of adding indoximod to standard 7+3 induction and high-dose cytarabine (HiDAC) consolidation chemotherapy for adult patients with newly diagnosed AML. The presentation highlighted an initial safety profile indicating that the treatment regimen was well tolerated with adverse events commensurate with chemotherapy alone. Evidence of clinical activity was observed for indoximod plus chemotherapy in newly diagnosed AML as supported by these Phase 1 data showing post-induction minimal residual disease (MRD) negativity rate of 86% and post-HiDAC1 MRD negativity of 100%.
"These data demonstrate the promising potential for indoximod in combination therapy for patients with newly diagnosed AML and the use of MRD status as a study endpoint,” said Dr. Ashkan Emadi. "We remain encouraged and look forward to additional data as this study proceeds.”
Fifty-seven patients were screened, and 38 patients initiated induction therapy on protocol. Five patients never received indoximod resulting in an intent-to-treat (ITT) population of 33 patients. Twenty-two patients received the pre-specified 80% of indoximod dosing required to be included in the per protocol (PP) analysis, 8 received less than 80% of the scheduled indoximod dosage, and 3 patients remained on induction treatment as of the date of data cut off. Of these 22 PP patients, 16/22 (73%) achieved complete morphological response (CR) and 6 were primary refractory. Of the patients who achieved CR, 14 had results available from MRD testing post-induction. MRD negativity was defined by a flow cytometry assay at a level of < 0.02% (Hematologics, Inc., Seattle, WA). Of those tested, 12/14 (86%) were MRD-negative. Of the 14 patients, 1 patient proceeded to transplant, and 13 began HiDAC consolidation therapy. Post-HiDAC consolidation, all 13 patients were tested for MRD status with all 13/13 (100%) reported to be MRD-negative. When benchmarked against available published studies, these initial data appear encouraging. For a more precise comparison, a contemporaneous multi-institutional dataset is being aggregated to benchmark these data against data generated from patients undergoing the same chemotherapy regimen without the addition of indoximod using the same MRD assay assessed at the same reference laboratory.
Safety data from this Phase 1 trial indicate that the combination therapy regimen was well tolerated. No RLTs were observed when combining indoximod with standard-of-care chemotherapy. Grade 3 or greater adverse hematologic events included febrile neutropenia, anemia, and thrombocytopenia while non-hematologic events included hypoxia, anemia, and pneumonia. The overall adverse event profile observed in this small sample size is consistent with that of 7+3 induction chemotherapy plus HiDAC consolidation alone.
About AML1,2
Adult acute myeloid leukemia (AML) is a cancer of the blood and bone marrow in which the bone marrow makes abnormal types of white blood cells, red blood cells, or platelets. AML is the most common type of acute leukemia in adults and tends to progress rapidly without treatment. In the US, approximately 19,000 patients per year are diagnosed with AML with only around 25% expected to survive longer than three years. Of those newly diagnosed patients, approximately half are categorized as young and fit for an aggressive chemotherapy treatment regimen.
1 National Cancer Institute
2 American
Society of Clinical Oncology
About Indoximod
Indoximod is an investigational, orally available small molecule targeting the IDO pathway. The IDO pathway is a key immuno-oncology target, suppressing immune response and allowing for immune escape by degrading tryptophan with the resultant production of kynurenine. Indoximod reverses the immunosuppressive effects of low tryptophan and high kynurenine through mechanisms that include modulation of the AhR-driven transcription of genes that control immune function. This results in increased proliferation of effector T cells, increased differentiation into helper T cells rather than regulatory T cells, and downregulation of IDO expression in dendritic cells. Indoximod is being evaluated in combination with treatment regimens including chemotherapy, radiation, checkpoint blockade and cancer vaccines across multiple indications including recurrent pediatric brain tumors, DIPG, and AML.
About NewLink Genetics Corporation
NewLink Genetics is a clinical stage biopharmaceutical company focusing on developing novel immuno-oncology product candidates to improve the lives of patients with cancer. NewLink Genetics' IDO pathway inhibitors are designed to harness multiple components of the immune system to combat cancer. For more information, please visit www.newlinkgenetics.com and follow us on Twitter @NLNKGenetics.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements of NewLink Genetics that involve substantial risks and uncertainties. All statements contained in this press release are forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words "may," "appear to,” "has potential to,” "look forward to,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements about results of NewLink’s clinical trials for product candidates and any other statements other than statements of historical fact. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that NewLink Genetics makes due to a number of important factors, including those risks discussed in "Risk Factors" and elsewhere in NewLink Genetics' Annual Report on Form 10-K for the year ended December 31, 2017 and other reports filed with the U.S. Securities and Exchange Commission (SEC). The forward-looking statements in this press release represent NewLink Genetics’ views as of the date of this press release. NewLink Genetics anticipates that subsequent events and developments will cause its views to change. However, while it may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. You should, therefore, not rely on these forward-looking statements as representing NewLink Genetics' views as of any date subsequent to the date of this press release.
View source version on businesswire.com: https://www.businesswire.com/news/home/20181202005047/en/
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu NewLink Genetics Corp.mehr Nachrichten
| Keine Nachrichten verfügbar. |