|
07.05.2020 22:01:00
|
Neurocrine Biosciences Announces New Data Published from the Largest Real-World Screening Study, RE-KINECT, Demonstrating that Movements Consistent with Tardive Dyskinesia Occur Frequently and Can...
SAN DIEGO, May 7, 2020 /PRNewswire/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced the publication of new data from RE-KINECT, the largest ever real-world screening study of patients with clinician-confirmed possible tardive dyskinesia (TD) demonstrating that the involuntary movements associated with TD can reduce health-related quality of life in patients living with psychiatric disorders.1 Based on clinical assessments, 28% of the 739 patients in this study had clinician-confirmed possible TD and 75% of patients in this group affirmed that they have felt self-conscious or embarrassed about involuntary movements that they could not seem to control.1 In addition, more than 40% of patients with possible TD reported that involuntary movements had "some" or "a lot" of impact on their ability to continue usual activities, such as talking, socializing and being productive.1 These data were recently published in the Journal of Clinical Psychopharmacology and demonstrate the importance of raising awareness of tardive dyskinesia and its impact on patients, especially during Mental Health Month and Tardive Dyskinesia Awareness Week (May 3–9).
"The real-world data from the RE-KINECT study are valuable for informing treatment decisions in clinical practice and demonstrate the importance of assessing the impact of involuntary movements from possible tardive dyskinesia on quality of life and daily functioning," said Stanley N. Caroff, M.D., Professor of Psychiatry at the University of Pennsylvania Perelman School of Medicine and the Corporal Michael J. Crescenz VA Medical Center in Philadelphia. "When screening and diagnosing patients with possible tardive dyskinesia, it may be informative to ask patients whether involuntary movements from possible tardive dyskinesia have had any impact on their ability to continue usual activities, be productive, take care of oneself, or socialize. For patients with visible or reported involuntary movements in the face or mouth, questions about their ability to talk, eat, and breathe might also be helpful. It is important to include assessments from patients and caregivers on a patient's ability to perform daily activities as there is clearly a negative social impact of the stigmatizing movements of tardive dyskinesia."
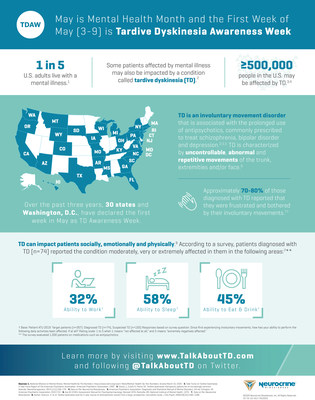
In the U.S., nearly one in five adults live with mental illness.2 Many people living with mental illness may also suffer from TD, an involuntary movement disorder that is associated with the prolonged use of antipsychotics, commonly prescribed to treat schizophrenia, bipolar disorder and depression.3–5 It is estimated that TD may affect at least 500,000 people in the U.S.5,6
"The timely publication of the real-world RE-KINECT study during Mental Health Month is important as it aims to uncover the emotional and physical challenges that people experience living with a mental illness such as schizophrenia, bipolar disorder and depression while also managing the devastating effects of involuntary movements from possible tardive dyskinesia on their daily lives," said Eiry W. Roberts, M.D., Chief Medical Officer at Neurocrine Biosciences. "Many patients reported that involuntary movements from possible tardive dyskinesia impact their ability to talk, socialize and be productive, leaving many of them feeling self-conscious or embarrassed. These data support the continued need to raise awareness of involuntary movements from possible tardive dyskinesia and the importance of properly screening, diagnosing and helping to relieve the suffering that many of these patients are experiencing."
Neurocrine Biosciences is proud to support Mental Health Month, collaborate with advocacy groups, educate legislators, and provide new data to healthcare providers in order to raise awareness of the challenges of living with mental illness and tardive dyskinesia. Over the past three years, 30 states and Washington D.C. have shown their support for people living with TD by declaring the first full week in May (May 3–9, 2020) as TD Awareness Week. In addition to the state declarations, several advocacy groups are also recognizing and supporting those living with tardive dyskinesia, including the Alliance for Patient Access, American Brain Coalition, Caregiver Action Network, Depression and Bipolar Support Alliance, International Bipolar Foundation, Mental Health America, Movement Disorders Policy Coalition, National Alliance on Mental Illness, National Council for Behavioral Health, National Organization for Tardive Dyskinesia and Schizophrenia and Related Disorders Alliance, among many others.
As part of Neurocrine Biosciences' commitment to TD education, resources are available at www.TalkAboutTD.com to help patients and caregivers understand TD and recognize its symptoms, learn about available support resources and have a conversation with their healthcare provider about ways to manage their TD.
About the RE-KINECT Study
RE-KINECT, a prospective real-world screening study that included 739 patients from 37 outpatient psychiatry practices in the U.S., was conducted with support from Neurocrine Biosciences. The study objective was to assess the presence and impact of possible tardive dyskinesia (TD) and describe the associated disease burden in a cohort of patients with one or more psychiatric disorders and a cumulative lifetime exposure to antipsychotic medication of three months or more. Patients were clinically evaluated for abnormal involuntary movements in general body regions (head/face, neck/trunk, upper/lower limbs) as well as for possible TD. Demographics, psychiatric history and medication history were captured as part of a 12-month retrospective chart review. Health-related quality of life was evaluated using the EuroQoL 5 Dimensions (EQ-5D-5L) questionnaire, which includes five domains that are each scored on a scale of 1 ("no problems") to 5 ("unable to perform") and the Sheehan Disability Scale (SDS), which is a brief patient-rated measure for disability and impairment, that includes three domains that are scored on a scale of 0 "not at all" to 10 "extremely."
About Tardive Dyskinesia (TD)
Tardive dyskinesia (TD) is a movement disorder that is characterized by uncontrollable, abnormal and repetitive movements of the face, torso and/or other body parts, which may be disruptive and negatively impact patients. The condition is caused by prolonged use of treatments that block dopamine receptors in the brain, such as antipsychotics commonly prescribed to treat mental illnesses such as schizophrenia, bipolar disorder and depression and certain anti-nausea medications. In patients with TD, these treatments are thought to result in irregular dopamine signaling in a region of the brain that controls movement. The symptoms of TD can be severe and are often persistent and irreversible. TD is estimated to affect at least 500,000 people in the U.S.
About Neurocrine Biosciences
Neurocrine Biosciences is a neuroscience-focused, biopharmaceutical company with 28 years of experience discovering and developing life-changing treatments for people with serious, challenging and under-addressed neurological, endocrine and psychiatric disorders. The company's diverse portfolio includes FDA-approved treatments for tardive dyskinesia, Parkinson's disease and endometriosis* and clinical development programs in multiple therapeutic areas including a gene therapy for Parkinson's disease, chorea in Huntington disease, congenital adrenal hyperplasia, epilepsy, uterine fibroids* and polycystic ovary syndrome*. Headquartered in San Diego, Neurocrine Biosciences specializes in targeting and interrupting disease-causing mechanisms involving the interconnected pathways of the nervous and endocrine systems. For more information, visit neurocrine.com, and follow the company on LinkedIn. (*in collaboration with AbbVie)
Forward-Looking Statements
In addition to historical facts, this press release contains forward-looking statements that involve a number of risks and uncertainties. These statements include, but are not limited to, statements related to the benefits to be derived from the RE-KINECT study, treatment decisions for TD and the prevalence rates of TD in the U.S. Among the factors that could cause actual results to differ materially from those indicated in the forward-looking statements are: risks and uncertainties associated with the scale and duration of the COVID-19 pandemic and resulting global, national, and local economic and financial disruptions; risk and uncertainties related to any COVID-19 quarantines, shelter-in-place and similar government orders that are currently in place or that may be put in place in the future, including the impact of such orders on our business operations and the business operations of the third parties on which we rely; risks that clinical trial activities replicate previous clinical trial results or may not be predictive of real-world results or of results in subsequent clinical trials; and other risks described in the Company's periodic reports filed with the Securities and Exchange Commission, including without limitation the Company's quarterly report on Form 10-Q for the quarter ended March 31, 2020. Neurocrine disclaims any obligation to update the statements contained in this press release after the date hereof.
References:

![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/neurocrine-biosciences-announces-new-data-published-from-the-largest-real-world-screening-study-re-kinect-demonstrating-that-movements-consistent-with-tardive-dyskinesia-occur-frequently-and-can-reduce-quality-of-life-in-patient-301055244.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/neurocrine-biosciences-announces-new-data-published-from-the-largest-real-world-screening-study-re-kinect-demonstrating-that-movements-consistent-with-tardive-dyskinesia-occur-frequently-and-can-reduce-quality-of-life-in-patient-301055244.html
SOURCE Neurocrine Biosciences, Inc.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!