|
09.06.2022 14:33:00
|
LumiraDx Achieves CE Mark for COVID-19 Antigen Test on Amira, its New Low-Cost Testing System
- Next-generation antigen test system built on more than eight years of research and backed by industry leading microfluidic experts
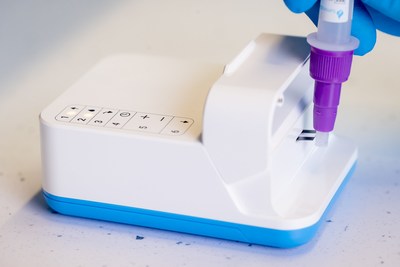
- The Amira System includes the Amira Analyzer, Amira SARS-CoV-2 Ag test strips, swabs and sample preparation materials
- Designed to provide lab-comparable performance at a lateral flow-comparable price
LONDON, June 9, 2022 /PRNewswire/ -- LumiraDx Limited (Nasdaq: LMDX), a next-generation point of care diagnostics company, today announced that it has achieved CE Mark for the Amira System, its new testing system that provides low-cost, highly sensitive COVID-19 testing. The Amira Analyzer reads the included Amira SARS-CoV-2 Ag test strips and returns a result in just 15 minutes. The Amira Analyzer condenses technology from large laboratory analyzers into a portable instrument, comparable in size and weight to a deck of playing cards, that delivers high performing testing in minutes. The initial CE Mark for the Amira System covers professional use at the point of care. However, the company plans to seek additional regulatory approvals and authorizations for the Amira System, including those required for non-professional, over the counter use, to provide value for home, events, workplace, and travel testing.
The microfluidic technology in the Amira SARS-CoV-2 Ag test delivers excellent testing performance. The test has a positive percent agreement of 90.00% and a negative percent agreement of 99.31% versus RT-PCR in symptomatic individuals, based on clinical data collected 0-10 days since symptom onset. Within this cohort, the Amira SARS-CoV-2 Ag test showed sensitivity of 95.6% up to a CT of 30 indicating high coverage of potentially infectious individuals.
Ron Zwanziger, LumiraDx's Chief Executive Officer commented, "The Amira System brings a unique combination of features to the market. It is one of the only COVID-19 testing solutions on the market that can offer highly accurate, objectively read results with the rapid turnaround time and low price of many lateral flow tests. Frequent testing has become a part of many of our lives in order to limit the spread of the virus. However, without trusted results, many testing protocols do not accurately protect patients, employees, and families."
The Amira System will be commercially available in Europe shortly as a starter kit with one analyzer and 200 Amira SARS CoV-2 Ag test strips.
About LumiraDxLumiraDx (Nasdaq: LMDX) is a next-generation point of care diagnostics company that is transforming community-based healthcare. Founded in 2014, LumiraDx manufactures and commercializes an innovative diagnostic Platform that supports a broad menu of tests with lab comparable performance at the point of care. LumiraDx diagnostic testing solutions are being deployed by governments and leading healthcare institutions across laboratories, urgent care, physician offices, pharmacies, schools, and workplaces to screen, diagnose, and monitor wellness as well as disease. LumiraDx has, on the market and in development, 30+ tests covering infectious diseases, cardiovascular diseases, diabetes, and coagulation disorders, all on the LumiraDx Platform. In addition, LumiraDx has a comprehensive portfolio of fast, accurate, and cost-efficient COVID-19 testing solutions from the lab to point of need.
LumiraDx is based in the UK with more than 1600 employees worldwide. Further information on LumiraDx is available at www.lumiradx.com
Cautionary Note Regarding Forward-Looking StatementsThis press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, including statements regarding the performance and benefits of the Amira System, the possibility of seeking additional regulatory approvals and authorizations for the Amira System, and the expected commercialization timeline for the Amira System. These statements involve risks, uncertainties and other factors that may cause actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements, including, among others, general economic, political and business conditions; regulatory changes; the ability of LumiraDx to maintain CE marking for its Amira Analyzer and Amira SARS-CoV-2 Ag test; and those factors discussed under the header "Risk Factors" in the Annual Report on Form 20-F for the year ended December 31, 2021, which was filed by LumiraDx with the Securities and Exchange Commission ("SEC") on April 13, 2022, and in other filings made by LumiraDx with the SEC. Although LumiraDx believes that it has a reasonable basis for each forward-looking statement contained in this press release, LumiraDx cautions you that these statements are based on a combination of facts and factors currently known by it and its projections of the future, about which it cannot be certain. LumiraDx undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise, except as required by applicable law.
Media Contact:
Colleen.McMillen@lumiradx.com
Commercial Contact:
Giffin.Daughtridge@lumiradx.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/lumiradx-achieves-ce-mark-for-covid-19-antigen-test-on-amira-its-new-low-cost-testing-system-301564940.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/lumiradx-achieves-ce-mark-for-covid-19-antigen-test-on-amira-its-new-low-cost-testing-system-301564940.html
SOURCE LumiraDx
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu LumiraDx Limited Registered Shs -A-mehr Nachrichten
| Keine Nachrichten verfügbar. |