|
11.07.2023 22:30:00
|
ivWatch Technology Demonstrates High Sensitivity in Wilhelmina Children's Hospital Study
Published in The Journal of Vascular Access (JVA), study shows company's patented patient monitoring system detects IV infiltrations and extravasations earlier than clinicians
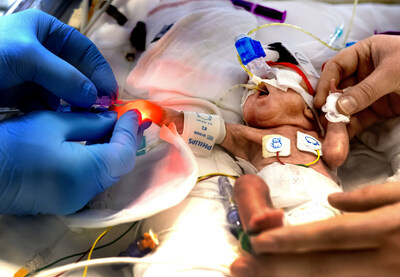
NEWPORT NEWS, Va., July 11, 2023 /PRNewswire/ -- A new study published in The Journal of Vascular Access (JVA) examines several important metrics that describe the performance of the ivWatch patient monitoring system for infiltrations and extravasations in a 24-bed neonatal intensive care unit (NICU) at Wilhelmina Children's Hospital, part of the University Medical Centre of Utrecht in the Netherlands. The continuous IV monitoring system was deployed to identify the leakage of infused fluids via short peripheral IV catheters (PIVC), which are commonplace with neonatal patients and are placed more than 200 times monthly in this NICU. This condition is referred to as peripheral IV infiltration/extravasation (PIVIE).
The study found that continuous infusion site monitoring using the ivWatch system detects PIVIE events earlier rather than relying on intermittent visual observation alone, and that detection occurred in 100% of leakage events prior to a clinician detecting such an event. During the monitoring time of the study with ivWatch technology, 11 infiltrations were detected in 21 monitored PIVCs, which corresponds to a 52.4% infiltration incidence rate. All infusates were vesicants and PIVIEs were graded as minor, indicating early detection of PIVIE.
The study was conducted using the PDSA (plan, do, study, act) model of quality improvement (QI) to provide a systematic framework for identifying PIVIE risks and evaluating the ivWatch model 400's continuous monitoring of PIVCs. From February 2018 to May 2019, a total of 3,476 procedures were observed to understand the seriousness of complications associated with PIVIE. In October 2022, the ivWatch model 400 was introduced, and it was used on 21 inserted PIVCs. This showed that the ivWatch system, which monitors infusion sites continuously, has the potential to detect PIVIE events earlier than the current standard of care.
Neonates are at high risk for unpredictable and unpreventable IV-therapy related complications due to their immature immune systems, fragile skin and blood vessels, and their exposure to additional invasive procedures.1 ivWatch technology uses a predictive algorithm and near-infrared light to see changes in the optical properties of the tissue around an IV site and notifies clinicians to check the IV site if changes are detected in real time.
The ivWatch system has been clinically tested in laboratory and real-world settings, demonstrating high sensitivity and specificity across various patient populations. The system's continuous monitoring and early detection capabilities have the potential to significantly reduce the severity of IV infiltrations and extravasations, improving patient outcomes and reducing healthcare costs.
The study concluded: "Continuous infusion site monitoring using the ivWatch [system] suggests this technology offers the potential to detect PIVIE events earlier than relying on intermittent observation alone (i.e. the current standard of care)."2
The study, entitled "Peripheral intravenous therapy infiltration/extravasation (PIVIE) risks and the potential for earlier notification of events using a novel sensor technology in a neonatal population," was authored by Matheus FPT van Rens, Daniel Vijlbrief, Sophie Braun, Kevin Hugill, Fredericus HJ van Loon, and Agnes van de Hoogen. The article can be viewed at https://journals.sagepub.com/doi/10.1177/11297298231185536.
To learn more about ivWatch, visit www.ivWatch.com.
1van Rens MFPT, Hugill K, Mahmah MA, et al. Evaluation of unmodifiable and potentially modifiable factors affecting peripheral intravenous device-related complications in neonates: a retrospective observational study. BMJ Open 2021; 11: e047788.
2van Rens MF, Vijlbrief D, Braun S, Hugill K, van Loon FH, van de Hoogen A. Peripheral intravenous therapy infiltration/extravasation (PIVIE) risks and the potential for earlier notification of events using a novel sensor technology in a neonatal population. The Journal of Vascular Access. 2023;0(0). doi:10.1177/11297298231185536.
Photo credit: Klaas Jan van der Weij
About ivWatch, LLC
ivWatch, LLC is a biosensor technology company focused on improving patient safety and the effectiveness of intravenous therapy. Our dedicated and passionate team is pioneering the use of optical sensors to detect adverse IV events early to minimize the risk of injury caused by infiltrations and extravasations. By using this technology, clinicians can leverage continuous monitoring to help identify infiltrations as early as possible. Our innovative IV monitoring solutions are backed by decades of clinical research and device development. To learn more, follow us on Twitter @ivWatch, Facebook @ivWatchLLC, Instagram @ivWatchLLC and LinkedIn @ivWatch-LLC, or visit www.ivWatch.com.
Contact: Erin Wendell, 410.952.3800, erin.wendell@ivwatch.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/ivwatch-technology-demonstrates-high-sensitivity-in-wilhelmina-childrens-hospital-study-301874821.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/ivwatch-technology-demonstrates-high-sensitivity-in-wilhelmina-childrens-hospital-study-301874821.html
SOURCE ivWatch, LLC
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!