|
04.07.2018 22:12:00
|
Health Canada Approves ERLEADA™* (apalutamide tablets), the First Treatment for Men with Non-Metastatic Castration-Resistant Prostate Cancer
ERLEADA™ decreased the risk of metastasis or death in patients by 70 per cent and improved median metastasis-free survival by more than two years1
TORONTO, July 4, 2018 /CNW/ - The Janssen Pharmaceutical Companies of Johnson & Johnson announced today that Health Canada has approved ERLEADA™ (apalutamide tablets), an oral treatment for patients with non-metastatic castration-resistant prostate cancer (nmCRPC).2 ERLEADA™ was granted Priority Review status by Health Canada and is the first treatment approved for men diagnosed with nmCRPC.

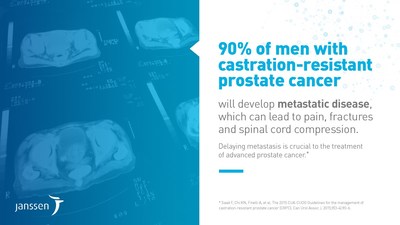
Prostate cancer is the most common cancer among Canadian men, with approximately 21,300 men diagnosed in 2017.3 Non-metastatic castration-resistant prostate cancer refers to patients who continue to show disease progression despite androgen deprivation therapy (ADT), and in whom the cancer has not spread to other parts of the body (metastasized).4 Nine out of 10 patients with castration-resistant prostate cancer will ultimately develop bone metastases, which can lead to pain, fractures and spinal cord compression.5 Delaying metastasis is crucial to the treatment of advanced prostate cancer.
Health Canada's approval of ERLEADA™ is based on Phase 3 data from the SPARTAN clinical trial, which showed ERLEADA™ decreased the risk of metastasis or death in nmCRPC patients by 70 per cent and improved median metastasis-free survival by more than two years (difference of 24.8 months) compared to placebo.6
"Previously, men with prostate cancer who were no longer responding to current therapies had to wait until the cancer started to spread before they could go on another treatment," said Dr. Bobby Shayegan, Head, Division of Urology at McMaster University, Hamilton, Ontario, and SPARTAN clinical investigator.** "ERLEADA™ has shown a meaningful delay in the progression to metastases along with a tolerable safety profile. I am excited to have an option I can offer my patients earlier than anything else that has been available."
ERLEADA™ is an orally administered next generation androgen receptor (AR) inhibitor that helps block the activity of androgens (hormones like testosterone) and has been shown to slow the progression of the disease.7 ERLEADA™ is used to treat men with prostate cancer who no longer respond to medical or surgical treatment that lowers testosterone before the cancer has spread to other parts of the body.8
"Until now, men living with this stage of prostate cancer have been forced to wait for their condition to worsen in order to be eligible for the next therapy," said Dr. Stuart Edmonds, Vice President, Research, Health Promotion and Survivorship, Prostate Cancer Canada. "The introduction of drugs to treat non-metastatic castration-resistant prostate cancer provides an important opportunity for these men and their families to maintain their quality of life, by being able to access an approved treatment that can delay disease progression."
About the SPARTAN Clinical Trial
The SPARTAN trial, published in the New England Journal of Medicine, randomized a total of 1,207 patients diagnosed with non-metastatic castration-resistant prostate cancer (nmCRPC) at a 2:1 ratio to receive either ERLEADA™ orally at a once daily dose of 240 mg (n=806) or placebo (n=401).9 All patients in the SPARTAN trial continued to receive androgen deprivation therapy (ADT).10 The double-blind, placebo-controlled trial measured the efficacy and safety of ERLEADA™ in patients with nmCRPC who had a rapidly rising prostate-specific antigen (PSA) while receiving continuous ADT.11 The trial was conducted at 332 sites in 26 countries, including sites in 31 Canadian cities.12
The majority of adverse events reported were grade 1 or 2, and the rates of serious adverse events were similar in both the ERLEADA™ and placebo groups (24.8 per cent and 23.1 per cent, respectively).13 The most common adverse events associated with ERLEADA™ were fatigue, hypertension, rash, diarrhea, nausea, weight loss, joint pain and falls.14 Eleven per cent of patients treated with ERLEADA™ discontinued treatment due to adverse events, compared to 7 per cent treated with placebo.15
The evaluation of ERLEADA™ was conducted under a unique pilot work-sharing initiative between Health Canada and Australian health regulator, the Therapeutic Goods Administration (TGA). This was a first-time collaboration between the two health regulators for the evaluation of a new chemical entity.
About the Janssen Pharmaceutical Companies of Johnson & Johnson
At the Janssen Pharmaceutical Companies of Johnson & Johnson, we are working to create a world without disease. Transforming lives by finding new and better ways to prevent, intercept, treat and cure disease inspires us. We bring together the best minds and pursue the most promising science.
We are Janssen. We collaborate with the world for the health of everyone in it. Learn more at www.janssen.com/canada. Follow us at @JanssenCanada. Janssen Inc. is part of the Janssen Pharmaceutical Companies of Johnson & Johnson.
*All trademark rights used under license.
**Dr. Shayegan was not compensated for any media work. He has been a paid consultant to Janssen Inc.
References
________________________
1 Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018.
2 ERLEADA™ Canadian Product Monograph, July 3, 2018.
3 Prostate Cancer Canada. Statistics. Available at: http://www.prostatecancer.ca/Prostate-Cancer/About-Prostate-Cancer/Statistics. Accessed April 19, 2018.
4 Saad F. Management of castration-resistant prostate cancer: a global approach. Curr Oncol. 2012;19(Suppl 3):S32-6.
5 Saad F, Chi KN, Finelli A, et al. The 2015 CUA-CUOG Guidelines for the management of castration-resistant prostate cancer (CRPC). Can Urol Assoc J. 2015;9(3-4):90-6.
6 Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018.
7 Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018.
8 ERLEADA™ Canadian Product Monograph, July 3, 2018.
9 Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018.
10 Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018.
11 Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018.
12 Aragon Pharmaceuticals, Inc. (2018). A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase III Study of ARN-509 in Men with Non-Metastatic (M0) Castration-Resistant Prostate Cancer. U.S. Library of National Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT01946204. Accessed April 3, 2018.
13 Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018.
14 ERLEADA™ Canadian Product Monograph, July 3, 2018.
15 ERLEADA™ Canadian Product Monograph, July 3, 2018.

SOURCE Janssen Inc.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!