|
03.06.2019 13:00:00
|
Findings from Can-Fite’s Phase II Liver Cancer Study were Presented at ASCO Annual Meeting Late-Breaking Session
Can-Fite BioPharma Ltd. (NYSE MKT: CANF) (TASE:CFBI), a biotechnology company with a pipeline of proprietary small molecule drugs that address cancer, liver and inflammatory diseases, announced today that data from its recently completed Phase II trial in patients with hepatocellular cancer (HCC), the most common form of liver cancer, was presented at the late-breaking abstract session of the 54th Annual Meeting of the American Society of Clinical Oncology (ASCO), the world’s largest clinical cancer research meeting.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190603005396/en/
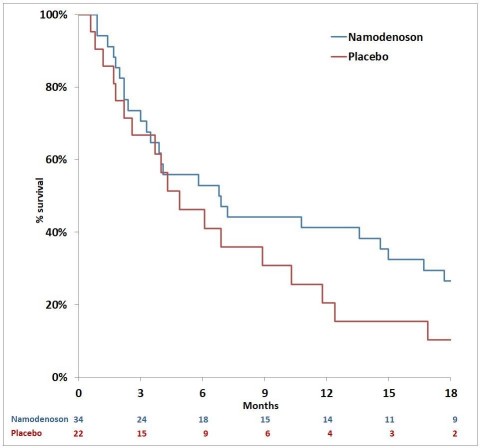
Figure 1 (Photo: Business Wire)
Prof. Salomon M. Stemmer, Principal Investigator of Can-Fite’s Phase II trial, delivered the presentation on Sunday June 2, 2019 at the Developmental Immunotherapy and Tumor Immunobiology session. The presentation is entitled: "A phase II, randomized, double-blind, placebo-controlled trial evaluating efficacy and safety of namodenoson (CF102), an a3 adenosine receptor agonist (A3AR), as a second-line treatment in patients with Child-Pugh B (CPB) advanced hepatocellular carcinoma (HCC).” While the Phase II study did not achieve its primary endpoint of overall survival in the whole population (n=78), superiority in overall survival was found in the largest study subpopulation of patients who were classified Child Pugh B7 (n=56) based on severity of disease compared to the placebo treated group. Median survival in the Namodenoson group (n = 34) was 6.8 months, versus 4.3 months for the placebo group (n = 18) (Hazard Ratio = 0.77, p = 0.40). (See Figure 1 )
The most impressive finding was that 44% of the patients with Child Pugh B7 treated with Namodenoson were alive at one year compared to 18% in the placebo group. In the overall patient population, among patients who had at least one assessment post baseline, disease control was significant in the Namodenoson group, 26% versus 10% in the control group after four months of treatment, P value 0.013. Among the other positive findings that were presented is the 9% partial response in the Namodenoson treated group vs. 0% in the placebo group. An example of a patient demonstrating an excellent tumor shrinkage was presented. (See Figure 2)
A3AR expression level at baseline was 1.98±0.36 in comparison to 1 unit in healthy subjects and was not changed substantially during the treatment period, demonstrating that continuous treatment with Namodenoson does not result in de-sensitization or loss of the target. Consistent with its previously demonstrated favorable safety profile, Namodenoson showed an adverse event profile that was comparable to that of placebo.
"No systemic therapies have shown clinical benefit in Child Pugh B patients with advanced HCC, underscoring the importance of developing an effective drug to serve this unmet medical need,” stated Can-Fite CEO Pnina Fishman. "We believe evidence of Namodenoson’s efficacy to prolong life in Child Pugh B7 liver cancer patients, without any deterioration in quality of life due to the drug’s strong safety data, make it a promising anti cancer agent to be moved into Phase III. We were pleased that ASCO selected Prof. Stemmer’s presentation so he could share these important findings at ASCO with the world’s leading clinical cancer researchers and physicians.”
About Namodenoson
Namodenoson is a small orally bioavailable drug that binds with high affinity and selectivity to the A3 adenosine receptor (A3AR). Namodenoson is being evaluated as a second line treatment for hepatocellular carcinoma, with a recently completed Phase II trial and planned Phase III trial in this indication. The drug is currently in an ongoing Phase II trial as a treatment for non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). A3AR is highly expressed in diseased cells whereas low expression is found in normal cells. This differential effect accounts for the excellent safety profile of the drug.
About Can-Fite BioPharma Ltd.
Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CFBI) is an advanced clinical stage drug development Company with a platform technology that is designed to address multi-billion dollar markets in the treatment of cancer, inflammatory disease and sexual dysfunction. The Company's lead drug candidate, Piclidenoson, is currently in Phase III trials for rheumatoid arthritis and psoriasis. Can-Fite's liver cancer drug, Namodenoson, recently completed a Phase II trial for hepatocellular carcinoma (HCC), the most common form of liver cancer, and is in a Phase II trial for the treatment of non-alcoholic steatohepatitis (NASH). Namodenoson has been granted Orphan Drug Designation in the U.S. and Europe and Fast Track Designation as a second line treatment for HCC by the U.S. Food and Drug Administration. Namodenoson has also shown proof of concept to potentially treat other cancers including colon, prostate, and melanoma. CF602, the Company's third drug candidate, has shown efficacy in the treatment of erectile dysfunction in preclinical studies and the Company is investigating additional compounds, targeting A3AR, for the treatment of sexual dysfunction. These drugs have an excellent safety profile with experience in over 1,000 patients in clinical studies to date. For more information please visit: www.can-fite.com.
Forward-Looking Statements
This press release may contain forward-looking statements, about Can-Fite’s expectations, beliefs or intentions regarding, among other things, market risks and uncertainties, its product development efforts, business, financial condition, results of operations, strategies or prospects. In addition, from time to time, Can-Fite or its representatives have made or may make forward-looking statements, orally or in writing. Forward-looking statements can be identified by the use of forward-looking words such as "believe,” "expect,” "intend,” "plan,” "may,” "should” or "anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters. These forward-looking statements may be included in, but are not limited to, various filings made by Can-Fite with the U.S. Securities and Exchange Commission, press releases or oral statements made by or with the approval of one of Can-Fite’s authorized executive officers. Forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause Can-Fite’s actual results to differ materially from any future results expressed or implied by the forward-looking statements. Many factors could cause Can-Fite’s actual activities or results to differ materially from the activities and results anticipated in such forward-looking statements. Factors that could cause our actual results to differ materially from those expressed or implied in such forward-looking statements include, but are not limited to: our history of losses and needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all; uncertainties of cash flows and inability to meet working capital needs; the initiation, timing, progress and results of our preclinical studies, clinical trials and other product candidate development efforts; our ability to advance our product candidates into clinical trials or to successfully complete our preclinical studies or clinical trials; our receipt of regulatory approvals for our product candidates, and the timing of other regulatory filings and approvals; the clinical development, commercialization and market acceptance of our product candidates; our ability to establish and maintain strategic partnerships and other corporate collaborations; the implementation of our business model and strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and our ability to operate our business without infringing the intellectual property rights of others; competitive companies, technologies and our industry; statements as to the impact of the political and security situation in Israel on our business; and risks and other risk factors detailed in Can-Fite’s filings with the SEC and in its periodic filings with the TASE. In addition, Can-Fite operates in an industry sector where securities values are highly volatile and may be influenced by economic and other factors beyond its control. Can-Fite does not undertake any obligation to publicly update these forward-looking statements, whether as a result of new information, future events or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20190603005396/en/
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu Can Fite Biopharma Ltd (spons. ADRs)mehr Nachrichten
| Keine Nachrichten verfügbar. |