|
29.12.2022 19:44:00
|
ABM RESPIRATORY CARE ANNOUNCES THE FDA CLEARANCE OF THE BIWAZE CLEAR SYSTEM
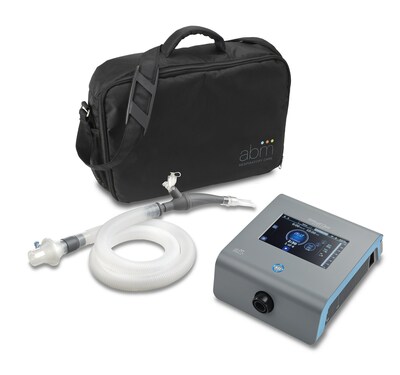
EAGAN, Minn., Dec. 29, 2022 /PRNewswire/ -- ABM Respiratory Care, a medical technology company focused on developing and globally commercializing novel integrated airway clearance and ventilation solutions, announced today the U.S. Food and Drug Administration (FDA) 510(k) clearance of the BiWaze® Clear System. This new airway clearance system helps patients clear their airways as well as prevent or treat atelectasis by using Oscillating Lung Expansion (OLE) therapy. BiWaze® Clear can be used to treat both acute and chronic respiratory conditions in the hospital, long-term care and home environments.
OLE therapy is a treatment used to help clear secretions from the airways in people with respiratory conditions such as cystic fibrosis, bronchiectasis and neuromuscular conditions. It has been utilized in the hospitals for over a decade and has been proven to be an effective airway clearance therapy. A recent study involving post-surgical patients demonstrated that OLE therapy decreased postoperative pulmonary complications by 31% and shortened the mean length of stay in the hospital by 1.6 days 1.
The innovative BiWaze® Clear system is portable and can be used anywhere, thanks to its lightweight and battery-powered design. It delivers OLE therapy using a unique Dual Lumen Breathing Circuit™, which prevents exhaled aerosol from escaping the handset or breathing tube until it is filtered by a coaxial bacterial/viral filter. BiWaze® Clear delivers three therapies: lung expansion, high frequency oscillation and nebulization with the Aerogen® Solo.
"We are continuing to expand our portfolio of respiratory care products to help more people with our technologies" said the CEO of ABM Respiratory Care, Vinay Joshi. "The BiWaze Clear System has been designed to help prevent the spread of bacterial and viral infections like COVID-19. We are proud to introduce this innovative OLE therapy system."
About ABM Respiratory Care
Founded in 2017, ABM Respiratory Care is dedicated to advancing patient care by developing intelligent, clinically differentiated and innovative respiratory care solutions to help people breathe better inside and outside the hospital. Our connected platform is designed to improve respiratory therapy by providing deeper breathing, improved oxygen exchange, reduce aerosol emission exposure, and communication for better disease management for people with compromised respiratory systems, in any care setting around the world. For more information visit, www.abmrc.com.
1. Huynh TT, Liesching TN, Cereda M, Lei Y, Frazer MJ, Nahouraii MR, Diette GB, Efficacy of Oscillation and Lung Expansion in Reducing Postoperative Pulmonary Complication, Journal of the American College of Surgeons (2019)
Investor and Media Contact:
Leah Noaeill
VP of Marketing and Clinical Affairs, ABM Respiratory Care
Leah.Noaeill@ABMRC.com
1.877.226.7201
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/abm-respiratory-care-announces-the-fda-clearance-of-the-biwaze-clear-system-301711155.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/abm-respiratory-care-announces-the-fda-clearance-of-the-biwaze-clear-system-301711155.html
SOURCE ABM Respiratory Care
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!