|
17.12.2020 21:46:00
|
Self-Contained, Rapid ADEXUSDx® COVID-19 Antibody Fingerstick Test Receives CE Mark for Deployment Across European Union
SPRINGDALE, Ark., Dec. 17, 2020 /PRNewswire/ -- Effective December 8, 2020, NOWDiagnostics, Inc. has received Conformité Européene (CE) mark approval for its ADEXUSDx® COVID-19 'antibody fingerstick' Test for use across 28 countries in the European Union (EU). The test first received CE mark approval for use in moderate/complex laboratory settings on July 28, 2020. The application for fingerstick use of the test was submitted November 2020 following completion of a Clinical Laboratory Improvement Amendments of 1988 (CLIA) Waiver trial in the United States (U.S.).
C19 Development, LLC, a wholly owned subsidiary of NOWDiagnostics, will begin offering the ADEXUSDx® COVID-19 Test for fingerstick use in a variety of health care settings in the EU—from clinics to hospital emergency rooms, while launching clinical trials of the test for use over the counter.
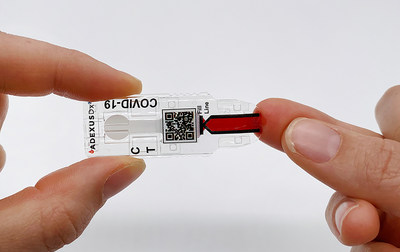
The ADEXUSDx® COVID-19 Test is a rapid serology, self-contained assay that measures the presence of SARS-CoV-2 antibodies to deliver accurate and reliable results in 15 minutes with no buffers, reagents, or additional equipment. The ADEXUSDx® COVID-19 Test has demonstrated a high level of analytical performance with 95.6% sensitivity (true positive rate) and 98.5% specificity (true negative rate). NOWDiagnostics applied to the U.S. Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of the test on May 29, 2020. The application is now under review.
"The ADEXUSDx® COVID-19 Test is literally a lab at the tip of your finger, specifically designed to make diagnostic testing possible anywhere. We are thrilled that the compelling performance data from our CLIA waiver clinical trial have resulted in expanded regulatory approval of the test as a fingerstick assay. We look forward to making this easy-to-use fingerstick test available for use in Europe first, then at home in the U.S., pending regulatory approval," said Kevin Clark, Chief Executive Officer of NOWDiagnostics, Inc.
The ADEXUSDx® COVID-19 Test uses the same platform as our other FDA-cleared and CE-marked, next-generation, easy-to-use tests which are affordable, portable, and deliver laboratory-quality results in minutes without any additional supplies. The test is developed and manufactured at NOWDiagnostics' Springdale, Arkansas, facility with materials sourced from U.S. suppliers and uses a drop (40 μL) of fingerstick or venous whole blood, plasma, or serum to detect the presence of antibodies to SARS-CoV-2, the virus that causes COVID-19.
This project has been funded in part with federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, Division of Research Innovation and Ventures under Contract No. 75A50120C00156.
NOWDiagnostics, Inc., based in Springdale, Arkansas, is a leader in innovative diagnostics testing. Its ADEXUSDx® product line features a lab at your fingertip, using a single drop of blood to test for a variety of common conditions, illnesses, and diseases, with results in a matter of minutes. By eliminating the need to send tests to off-site laboratories, NOWDiagnostics products have the potential to decrease the waiting period to determine test results by days. For more information about NOWDiagnostics, visit www.nowdx.com. For more information about the ADEXUSDx® COVID-19 Test, including its intended use, features, benefits and limitations, and directions for use, visit www.c19development.com. The ADEXUSDx® COVID-19 Test will be distributed by C19 Development, LLC, a wholly owned subsidiary of NOWDiagnostics. Laboratories may contact www.c19development.com/order to place an order.

![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/self-contained-rapid-adexusdx-covid-19-antibody-fingerstick-test-receives-ce-mark-for-deployment-across-european-union-301195579.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/self-contained-rapid-adexusdx-covid-19-antibody-fingerstick-test-receives-ce-mark-for-deployment-across-european-union-301195579.html
SOURCE NOWDiagnostics
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!